 INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
Unit Written By. Unit No. 1. Dr. K. S. Dhami (Ret. Proff.) 01 02
 Modern inorganic chemistry
Modern inorganic chemistry
Page 1. Modern inorganic chemistry. AN INTERMEDIATE TEXT. C. CHAMBERS B.Sc in first-year tertiary level chemistry courses. The new syllabuses have made it ...
 B. Sc. II YEAR INORGANIC CHEMISTRY-II
B. Sc. II YEAR INORGANIC CHEMISTRY-II
INORGANIC CHEMISTRY-II. BSCCH-201. UTTARAKHAND OPEN UNIVERSITY. Page 1. UNIT 1: CHEMISTRY OF THE ELEMENTS OF. FIRST TRANSITION (3-d) SERIES. CONTENTS: 1.1
 STATE MODEL SYLLABUS FOR UNDER GRADUATE COURSE IN
STATE MODEL SYLLABUS FOR UNDER GRADUATE COURSE IN
Chand Publisher. 2012. Reference Books: 1. Y R Sharma
 Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Department of Chemistry. Inorganic Chemistry (paper code - CH-201). B.Sc 1st Year (Sem. 2nd). GDC Memorial College. (Approved by Govt. of Haryana & Affiliated
 1 INDIRA GANDHI UNIVERSITY MEERPUR (REWARI)
1 INDIRA GANDHI UNIVERSITY MEERPUR (REWARI)
B. Sc IIIrd Semester. Paper IX (Theory) Inorganic Chemistry. Max. Marks: 29. CH-301. Time: 3 Hrs
 INORGANIC CHEMISTRY
INORGANIC CHEMISTRY
B.Sc. B.Sc. (Hons.) & M.Sc. Students of Indian Universities as also for Aqueous chemistry of copper (1) (680). Aqueous chemistry of Cu (II) (681).
 Kurukshetra University Kurukshetra Scheme and Syllabi for B.Sc
Kurukshetra University Kurukshetra Scheme and Syllabi for B.Sc
II Year (IIIrd Semester). Paper-VIII (CH-201) Inorganic Chemistry (Theory). M.Marks: 32. Time: 3 Hrs. Note: Nine questions will be set. Q.No.1 based on whole
 Concise Inorganic Chemistry (4th Edition)
Concise Inorganic Chemistry (4th Edition)
Aparl from ant fair dealing' for the purposes of resean:h 1.>r private study or criticism or review. as permitted under the UK Copyright Designs and. Patents
 B.Sc. III YEAR INORGANIC CHEMISTRY-III
B.Sc. III YEAR INORGANIC CHEMISTRY-III
As a mixture of biochemistry and inorganic chemistry bioinorganic chemistry is 8.3.1 Classification of inorganic polymers. 8.3.1 General properties of ...
 INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC CHEMISTRY-I. BSCCH-101. UTTARAKHAND OPEN UNIVERSITY. Page 1 (v) By the year 1900 some 30 more elements had been added to the list of elements ...
 Modern inorganic chemistry
Modern inorganic chemistry
in first-year tertiary level chemistry courses. the facts of inorganic chemistry and in this book the first four chap- ... (Liverpool B.Sc.
 B. SC. CHEMISTRY (Subsidiary)
B. SC. CHEMISTRY (Subsidiary)
B.Sc. COURSE PLAN (Subsidiary). Inorganic Chemistry. 1st Year. 1st Semester : Paper IS & Paper IIS (Group C each). Marks : 16 + 16.
 Notes of inorganic chemistry for BSc II
Notes of inorganic chemistry for BSc II
(1). T on the basis of this concept have been defined in torms of aqueous solutions. This defination is applicable for hydrogen and hydroxyl Compounds.
 B. Sc. II YEAR INORGANIC CHEMISTRY-II
B. Sc. II YEAR INORGANIC CHEMISTRY-II
Unit-1 Chemistry of elements of first transition series INORGANIC CHEMISTRY-II. BSCCH-201. UTTARAKHAND OPEN UNIVERSITY. Page 1 ... ion_compounds.pdf.
 Syllabus B.Sc.-Chemistry
Syllabus B.Sc.-Chemistry
[1]. V. B. S. Purvanchal University Jaunpur. Syllabus. B.Sc.-Chemistry. B.Sc.-1- 1. Inorganic Chemistry. Theoretical. 50. 3.00. 2. Organic Chemistry.
 MODEL PAPER FIRST YEAR B.Sc. DEGREE EXAMINATION
MODEL PAPER FIRST YEAR B.Sc. DEGREE EXAMINATION
MODEL PAPER. FIRST YEAR B.Sc. DEGREE EXAMINATION. SEMESTER-I. CHEMISTRY Course-I: INORGANIC & PHYSICAL CHEMISTRY. Time: 3 hours. Maximum Marks: 75.
 t- * rr1*
$---
t- * rr1*
$---
B. Sc. Ist Year (Ist Semester) paper I (Theory) Inorganic chemistry (cH-101) Max. Marks: 27. Time: 3 Hrs. N o t e: The examiners will set seven questions in
 Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101). B.Sc 1st Year (Sem. 1st). GDC Memorial College. (Approved by Govt. of Haryana & Affiliated to M D University
MODEL PAPER
FIRST YEAR B.Sc., DEGREE EXAMINATION
SEMESTER-I
CHEMISTRY Course-I: INORGANIC & PHYSICAL CHEMISTRYTime: 3 hours Maximum Marks: 75
PART- A 5 X 5 = 25 Marks
Answer any FIVE of the following questions. Each carries FIVE marks1. Explain the preparation & structures of Phosphonitrilic compounds.
2. Explain in brief, catalytic properties & stability of various oxidation states of dblock
elements.3. Write short note on Bravais lattices and crystal systems.
4. What are Smectic & Nematic liquid Crystals? Explain.
5. Write account on Common ion effect & Solubility product.
6.7. Explain Actinide Constraction.
8. Explain the structure of Borazine.
PART- B (Inorganic Chemistry) 2 X 10 = 20 Marks
Answer any two of the following questions. Each carries TEN marks9. Explain Classification, Preparations & uses of Silicones
10. (i) What are Pseudohalogens.
(ii) Explain the Structures of any one AX3 & AX5interhalogen compounds.11. What is Lanthanide Contraction? Explain the Consequences of Lanthanide
Contraction.
12. (i) Explain the magnetic properties of d- block elements.
(ii) Explain about Conductors, Semi-Conductors& Insulators using Band Theory.PART- C (Physical Chemistry) 3 X 10 = 30 Marks
Answer any three of the following questions. Each carries TEN marks13. Write an essay on Crystal defects.
14. method.15. Derive the relationship between Critical constants &Vanderwaal constants
16. (i) Write any 5 differences between liquid crystals & liquids, solids
(ii) Write the applications of Liquid crystals.17. Explain Nernst distribution Law. Explain its applications
18. What are colligative properties. Write experimental methods for determination of
molar mass of a non-volatile solute by using Elevation in boiling point & depression in freezing point.MODEL PAPER
FIRST YEAR B.Sc., DEGREE EXAMINATION
SEMESTER-II
CHEMISTRY COURSE -II: ORGANIC & GENERAL CHEMISTRY
Time: 3 hours Maximum Marks: 75
PART- A 5 X 5 = 25 Marks Answer any FIVE of the following questions. Each carries FIVE marks1. Write different conformations of n-butane. Explain their relative stability..
2. Explain 1,2- & 1,4- addition reactions of conjugated dienes.
3. Explain the orientation effect of halogens on mono substituted benzene.
4. Explain the mechanism of E1CB elimination reaction.
5. Explain the structure of ClF3 by Valency Bond theory.
6. What are Hard & soft acids & bases? Explain with examples.
7. Draw the Wedge, Fischer, Newmann& saw-Horse representations for Tartaric acid.
8. Define Enantiomers and Diastereomers and give two examples for each.
PART- B (Organic Chemistry) 3 X 10 = 30 Marks
Answer any three of the following questions. Each carries TEN marks9. .(i) Write the preparation of alkanes by Wurtz and Corey-House reaction.
(ii) Explain Halogenation of alkanes. Explain the reactivity and selectivity in free radical substitutions.10. (i) Explain Baeyer Strain Theory
(ii)Draw the conformations of Cyclohexane and explain their stability by drawing energy profile diagram.11. (i) Write any two methods of preparation of alkenes.
(ii) Explain the mechanism of Markownikiff and Anti-Markownikoff addition ofHBr to alkene.
12. (i) Explain the acidity of 1-alkynes
(ii) How will you prepare acetaldehyde and acetone from alkynes? (iii)Write alkylation reaction of terminal alkne.13. Define Huckel rule of aromatic compounds. What are benzenoid and nonbenzenoid
aromatic compounds? Give examples.14. Explain the mechanisms of Nitration and Friedel-
PART- C (General Chemistry) 2 X 10 = 20 Marks
Answer any two of the following questions. Each carries TEN marks15. (i) Define Hardy-Schulze rule & Gold number.
(ii) Differentiate Physisorption& Chemisorption. Explain Langmuir adsorption isotherm.16. Construct the Molecular Orbital diagram for O2 and NO and explain their bond order
and magnetic property.17. Define racemic mixture. Explain any two techniques for resolution of racemic mixture.
18. (i) Define Optical activity and Specific rotation.
(ii)Draw the R- & S- isomers of Alanine, Glyceraldehyde. (iii)Write the E- & Z- isomers of 2-butene.MODEL PAPER
SECOND YEAR B.Sc., DEGREE EXAMINATION
SEMESTER-III
CHEMISTRY COURSE-III: ORGANIC CHEMISTRY &
SPECTROSCOPY
Time: 3 hours Maximum Marks: 75
PART- A 5 X 5 = 25 Marks Answer any FIVE of the following questions. Each carries FIVE marks1. Discuss two methods for preparation of aryl halides.
2. Explain the mechanism for Pinacol-Pinacolone rearrangement.
3. Discuss the mechanism for Bayer-villiger oxidation reaction.
4. Explain the effect of substituents on acidic strength of mono-carboxylic
acids.5. Write the mechanism for Claisen Condensation reaction.
6. Write the selection rules in rotational spectroscopy.
7. Explain Spin Spin coupling and Coupling Constant.
8. Explain types of electronic transitions in UV spectroscopy.
PART- B (Organic Chemistry) 3 X 10 = 30 Marks Answer any three of the following questions. Each carries TEN marks9. Give the mechanism & stereochemistry of SN1& SN2 reactions of alkyl halides with
suitable example.10. Explain the following reactions with mechanism.
(i) Reimer-Tiemann reaction (ii) Fries rearrangement.11. Discuss the mechanism for following reactions.
(i) Perkin reaction. (ii) Cannizaro reaction12. Write the preparation and any three synthetic applications of diethyl malonate.
13. Explain acid and base hydrolysis reaction of esters with mechanism.
14. Explain the mechanisms of Curtius rearrangement & Arndt Eistert reaction.
PART- C (Spectroscopy) 2 X 10 = 20 Marks
Answer any two of the following questions. Each carries TEN marks15. (i) Write a note on vibrational degrees of freedom for polyatomic molecules.
(ii) Explain different modes of vibrations & selection rules in IR spectroscopy.16. (i) Define Bathochromic shift. Explain the effect of conjugation in U.V. spectroscopy.
(ii) Discuss the principle of NMR spectroscopy.17. Write Woodward-ȜĮȕ
unsaturated carbonyl compounds , and apply them for one example each.18. What is Fingerprint region. Explain its signigicance with an example.(ii) Write IR
spectral data for any one alcohol, aldehyde and ketoneMODEL PAPER
SECOND YEAR B.Sc., DEGREE EXAMINATION
SEMESTER-IV
CHEMISTRY COURSE -IV: INORGANIC, ORGANIC &
PHYSICAL CHEMISTRY
Time: 3 hours Maximum Marks: 75
PART- A 5 X 5 = 25 Marks Answer any FIVE of the following questions. Each carries FIVE marks1. Describe the 18 electron rule of mono nuclear and polynuclear metal
carbonyls with suitable examples.2. What are epimers and anomers. Give examples.
3. Discuss about iso electric point and zwitter ion.
4. Discuss the Paul-Knorr synthesis of five membered heterocyclic compounds.
5. Explain Tautomerism shown by nitro alkanes
6. Discuss the basic nature of amines.
7. Write the differences between thermal and photochemical reactions.
8. Derive heat capacities and derive Cp Cv = R
PART- B (Inorganic Chemistry) 1 X 10 = 10 Marks Answer any one of the following questions. Each carries TEN marks9. What are organometallic compounds? Discuss their Classification on the basis of type
of bonds with examples.10. Discuss the general methods of preparations of mono & bi-nuclear carbonyls of 3d
series. PART- C (Organic Chemistry) 3 X 10 = 30 Marks Answer any three of the following questions. Each carries TEN marks11. Discuss the constitution, configuration and ring size of glucose. Draw the Haworth and
Conformational structure of glucose.
12. (i
(ii) Explain Kiliani- Fischer synthesis.13. What are amino acids? Write any three general methods of preparation of amino acids.
14. Discuss the aromatic character of Furan, Thiophene and Pyrrole.
15. Write the mechanism for the following.
(i) Nef reaction (ii) Mannich reaction16. (i) Explain Hinsberg separation of amines.
(ii) Discuss any three synthetic applications of diazonium salts. PART- D (Physical Chemistry) 1 X 10 = 10 Marks Answer any one of the following questions. Each carries TEN marks17. What is quantum yield? Explain the photochemical combination of Hydrogen Chlorine
and Hydrogen - Bromine.18. Define entropy. Describe entropy changes in the reversible and irreversible process.
MODEL PAPER
SECOND YEAR B.Sc., DEGREE EXAMINATION
SEMESTER-IV
CHEMISTRY COURSE V: INORGANIC & PHYSICAL
CHEMISTRY
Time: 3 hours Maximum Marks: 75
PART- A 5 X 5 = 25 Marks
Answer any FIVE of the following questions. Each carries FIVE marks1. Write note on Jahn-Teller distortion.
2. Explain Labile & inert complexes.
3. 4.5. Explain any two conductometric titrations.
6. Write note on Fuel Cells with examples and applications.
7. What is enzyme catalysis? Write any three factors effecting enzyme
catalysis.8. Derive Michaels- Menten equation.
PART- B (Inorganic Chemistry) 2 X 10 = 20 Marks
Answer any two of the following questions. Each carries TEN marks9 Explain Valence Bond theory with Inner and Outer orbital complexes. Write
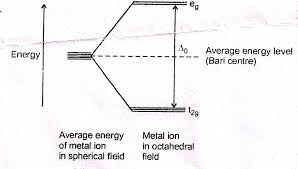
limitations of VBT.10. Define CFSE. Explain the factors effecting the magnitude of crystal field
splitting energy.11. Explain Trans effect. Explain the theories of trans effect and write any two
applications of trans effect.12. (i)Write the biological functions of Haemoglobin and Myoglobin.
(ii) Write note on use of chelating agents in medicines. PART- C (Physical Chemistry) 3 X 10 = 30 Marks Answer any three of the following questions. Each carries TEN marksquotesdbs_dbs7.pdfusesText_5[PDF] inscription administrative paris 7 paces
[PDF] inscription cnc 2020
[PDF] inscription concours ecricome 2020
[PDF] inscription dakar
[PDF] inscription faculté de médecine paris 7
[PDF] inscription licence psychologie ied paris 8
[PDF] inscription maternelle 4 ans laval
[PDF] insead
[PDF] insert into table sql
[PDF] insert numeric values sql
[PDF] inside listening and speaking pdf
[PDF] inside tokyo itinerary
[PDF] install fontspec package latex
[PDF] install fortigate
